Researchers have developed a new, more efficient, and environmentally friendly method for producing hydrogen peroxide, a chemical with a wide range of industrial applications. Hydrogen peroxide is commonly used as an oxidizing agent in various industries, including environmental disinfection, chemical synthesis, paper bleaching, and fuel cells.
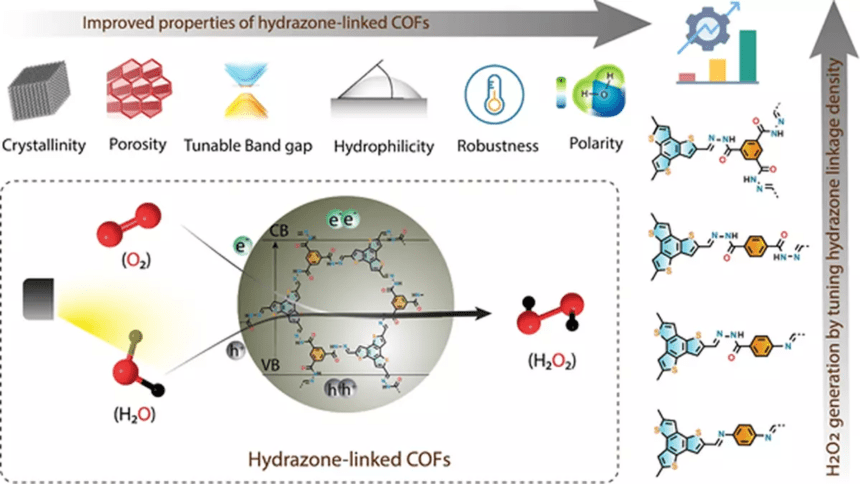
The current industrial production of hydrogen peroxide relies on the anthraquinone oxidation process, which is energy-intensive, costly, and generates hazardous by-products. Scientists are now exploring sustainable and economical ways to produce hydrogen peroxide from renewable sources. One promising approach involves the use of covalent organic frameworks (COFs), a class of porous polymers with customizable catalytic sites and light-harvesting properties in the visible spectrum.
Researchers at the SN Bose National Centre for Basic Sciences in Kolkata have designed a series of water-affinitive COFs by controlling the hydrazone linkage density. These COFs facilitate the photocatalytic generation of hydrogen peroxide by providing ample docking sites for water and oxygen, which promote the water oxidation reaction (WOR) and oxygen reduction reaction (ORR) – the two main pathways for hydrogen peroxide production.
Under sunlight irradiation, the hydrazone-linked COFs were able to produce a significant amount of hydrogen peroxide (550 micromoles per gram per hour), surpassing the performance of most organic photocatalysts under similar conditions. This demonstrates a clean and sustainable pathway for hydrogen peroxide synthesis.
Overall, this research offers a promising solution for producing hydrogen peroxide in an eco-friendly and cost-effective manner, with potential benefits for various industries and applications.